It can react vigorously, violently or explosively with oxidizers When Ethanol comes in contact with Platinum or Sodium, it liberates flammable hydrogen gas It can react vigorously or explosively with acid hydrides or acid chlorides It reacts with alkali metals to liberate flammable hydrogen gas It reacts with acetyl bromide to evolve hydrogen bromide It reacts with ammona + silver nitrate to form silver nitride and silver fulminate Ethyl alcohol can react with freshly cut/etched/scratched aluminum with the evolution of heat and release of hydrogen gas. The Ethyl alcohol has to be on the aluminum surface as it is being cut/scratched/etched
Ethyl Alcohol-Ethanol CAS 64-17-5
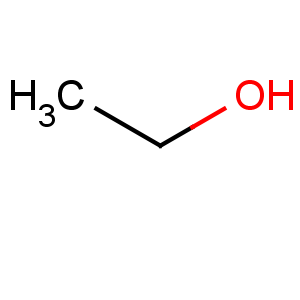
Ethanol is a chemical compound, a simple alcohol with the chemical formula C ₂H ₆O. Its formula can be also written as CH ₃−CH ₂−OH or C ₂H ₅OH, and is often abbreviated as EtOH. Ethanol is a volatile, flammable, colorless liquid with a slight characteristic odor.
Formula: C2H5OH
Molar mass: 46.07 g/mol
Boiling point: 78.37 °C
IUPAC ID: ethanol
Density: 789 kg/m³
Melting point: -114.1 °C